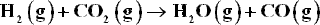
At high temperatures and low to moderate pressures, the reacting species form an ideal gas mixture. Application of the summability equation to Eq. (11.26) yields :

When the Gibbs energies of the elements in their standard states are set equal to zero, Gi = DGof,i for each species, and then :

At the beginning of Sec. 13.2, we noted that Eq. (14.64) is a criterion of equilibrium. Applied to the water-gas-shift reaction with the understanding that T and P are constant, this equation becomes :

Here, however, dn/dε = 0. The equilibrium criterion therefore becomes:

Once the yi are eliminated in favor of ε, Eq. (A) relates G to ε. Data for ΔGof,I for the compounds of interest are given with Ex 13.13. For a temperature of 1300 K (the reaction is unaffected by P) and for a feed of 1 mol H2 and 1 mol CO2:
a.) Determine the equilibrium value of ε by application of Eq. (B).
b.) Plot G vs. ε, indicating the location of the equilibrium value of ε determined in part (a).
2 comments:
I was checking my answers and I saw you didn't prepare a graph of G vs e. Do we need to prepare a graph of G vs. e?
Erik,
Yes, please do the plot. I just forgot to do it !
Post a Comment