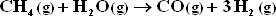
The only other reaction considered is :
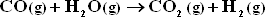
Assume equilibrium is attained for both reactions at 1 bar and 1300 K.
a.) Would it be better to carry out the reaction at pressures above 1 bar ?
b.) Would it be better to carry out the reaction at temperatures below 1300 K ?
c.) Estimate the molar ratio of H2 to CO in the synthesis gas if the feed consists of an equimolar mixture of steam and methane,
d.) Repeat part c) for a steam to methane mole ratio in the feed of 2.0.
e.) How could the feed composition be altered to yield a lower ratio of H2 to CO in the synthesis gas than is obtained in part c.) ?
f.) Is there any danger that carbon will deposit by the reaction :
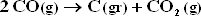
under conditions of part (c) ? Part d ? If so, how could the feed be altered to prevent carbon deposition ?
2 comments:
I came to the same conclusion you did about the decrease in temperature but when I used P = 100 bar my EOR1/EOR2 ratio was 77,595 i.e. the second reaction barely occurred. Can this possibly be right?
ahayles 4:23 PM
I considered pressures of 0.5, 0.9, 1, 1.1, 1.5 bar and calculated e1/e2. I found that in the neighborhood of 1 bar, increasing P decreased e1/e2. I did not try it for 100 bar. I just tried it at 100 bar and I got e1/e2 = 8...which is not good at all. I would say something is wrong with your work. Bring your Excel to office hours on Wed if you cannot get the problem solved in Quiz Section on Tue.
Post a Comment