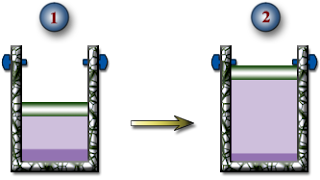
a.) The temperature of the R-134a when the piston reaches the stops.
b.) The boundary work done during this expansion process.
c.) The heat transfer during this expansion process.
d.) Show this process on a PV Diagram.
Learning undergraduate engineering thermodynamics might be less painful with a blog. I hope that students, faculty and interested observers will share their thoughts on the laws of thermodynamics, phase and chemical equilibrium and many related topics.

13 comments:
I calculated the temp. and boundary work but I can't figure out how to calculate Q12. In class you gave the equation as Q=m(delta)H, but what values do we use to look up H at stages 1 and 2?
Anon:
At stage 1 you know T = Tsat and you know Mliq and Mvap so you can calculate the quality and then H1.
At state 2, you know T and you can calculate Vhat2. These two will let you calculate H2. Then you can apply the 1st Law to the process to determine Q because Wb.
how is the initial volume calculated?
Anon:
Use the mass and the specific volume of each phase.
how do i go about calculating the temperature?
y:
Compare the specific volume to Vhat sat liquid and sat vapor. You will discover it lies between. This lets you conclude that T2 = Tsat.
I didn't really understand that last explanation on how to get the temperature... can you re-explain that?
Never mind on that last question...I understand now...thanks
how do we plot the PV diagram if the process is isothermal and isobaric at the same time?
I'm not seeing how to get H2 from T and Vhat2. Anybody have a clue?
Nutella:
I am glad you worked it out ! Good work.
Y:
Plot the 2-phase envelope and draw the relevant isotherm. This process is indeed isothermal and isobaric, so it moves along the horizontal portion of the isotherm on the PV Diagram.
Nutella:
Compare Vhat2 to Vsatliq and Vsatvap at T2 that you got in part (a). It turns out that Vhat2 falls between the two and, therefore, you need to determine the quality. Once you know the quality, use it to evaluate Hhat2.
Post a Comment