Please post any questions or discussion related to Ch 1 that don't relate directly to a HW problem. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Learning undergraduate engineering thermodynamics might be less painful with a blog. I hope that students, faculty and interested observers will share their thoughts on the laws of thermodynamics, phase and chemical equilibrium and many related topics.
Tuesday, March 27, 2007
Ch 1 - Any thing except HW
Please post any questions or discussion related to Ch 1 that don't relate directly to a HW problem. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
Wednesday, March 21, 2007
HW #2, P1- Steam Table Fundamentals - 4 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #2, P2- R-134a Table Fundamentals - 4 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #2, P3- R-134a Table Fundamentals - 4 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #2, P4- Isochoric Heating of Water - 4 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #2, P5- Isobaric Expansion of Water - 6 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #2, P6- Inflating an Automobile Tire - 6 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #2, P7- An Application of Equations of State - 8 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #2, P8- Relative Humidity and Fogged Glasses - 5 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #2, P9- Spray Dryer - 5 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #1, P1- Mass, Force, Density and Acceleration - 4 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #1, P2- Mass, Weight and Acceleration - 3 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #1, P3- NOx Emissions: UNITS - 3 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #1, P4- Temperature Conversions: Celsius to Fahrenheit - 2 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #1, P5- Temperature Conversions: Fahrenheit to Celsius - 2 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #1, P6- Temperature Change - 2 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #1, P7- Absolute and gauge Pressures - 5 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
HW #1, P8- Differential, Multi-Fluid Manometer - 6 pts
Please post any questions or discussion related to this problem as comments on this message. Feel free to answer other students' questions. I will check the blog M-F and once on the weekend.
Dr. B
Dr. B
Saturday, March 10, 2007
Final Exam
Please post here any questions you have about the final exam.
It has been a pleasure working with you.
Best of luck on the final exam !
Adios,
Dr. B
It has been a pleasure working with you.
Best of luck on the final exam !
Adios,
Dr. B
Friday, March 02, 2007
HW #6, P13.34 - Multiple Reaction Equilibria and Their Dependence on T and P - 22 pts
"Synthesis gas" may be produced by the catalytic reforming of methane with steam :
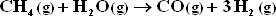
The only other reaction considered is :
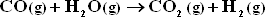
Assume equilibrium is attained for both reactions at 1 bar and 1300 K.
a.) Would it be better to carry out the reaction at pressures above 1 bar ?
b.) Would it be better to carry out the reaction at temperatures below 1300 K ?
c.) Estimate the molar ratio of H2 to CO in the synthesis gas if the feed consists of an equimolar mixture of steam and methane,
d.) Repeat part c) for a steam to methane mole ratio in the feed of 2.0.
e.) How could the feed composition be altered to yield a lower ratio of H2 to CO in the synthesis gas than is obtained in part c.) ?
f.) Is there any danger that carbon will deposit by the reaction :
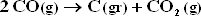
under conditions of part (c) ? Part d ? If so, how could the feed be altered to prevent carbon deposition ?
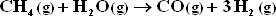
The only other reaction considered is :
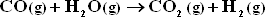
Assume equilibrium is attained for both reactions at 1 bar and 1300 K.
a.) Would it be better to carry out the reaction at pressures above 1 bar ?
b.) Would it be better to carry out the reaction at temperatures below 1300 K ?
c.) Estimate the molar ratio of H2 to CO in the synthesis gas if the feed consists of an equimolar mixture of steam and methane,
d.) Repeat part c) for a steam to methane mole ratio in the feed of 2.0.
e.) How could the feed composition be altered to yield a lower ratio of H2 to CO in the synthesis gas than is obtained in part c.) ?
f.) Is there any danger that carbon will deposit by the reaction :
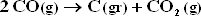
under conditions of part (c) ? Part d ? If so, how could the feed be altered to prevent carbon deposition ?
HW #6, P13.31 - Keq and γi for a Liquid Phase Isomerization Reaction - 10 pts
The following isomerization reaction occurs in the liquid phase :

where A and B are miscible liquids for which :

If ΔGo298 = -1,000 J/mole, what is the equilibrium composition of the mixture at 25oC ? How much error is introduced if one assumes that A and B form an ideal solution ?

where A and B are miscible liquids for which :

If ΔGo298 = -1,000 J/mole, what is the equilibrium composition of the mixture at 25oC ? How much error is introduced if one assumes that A and B form an ideal solution ?
HW #6, P13.24 - Independent Chemical Reactions and Degrees of Freedom - 4 pts
A chemically reactive system contains the following species in the gas phase: NH3, NO, NO2, O2, and H2O. Determine a complete set of independent reactions for this system. How many degrees of freedom does the system have ?
HW #6, P13.23 - Vapor Pressure of Decompositioon of NH4Cl(s) to NH3 and HCl - 12 pts
Ammonium Chloride [NH4Cl (s)] decomposes upon heating to yield a gas mixture of ammonia and hydrochloric acid. At what temperature does ammonium chloride exert a decomposition pressure of 1.5 bar ? For NH4Cl (s), ΔHof,298 = -314,430 J/mole and ΔGof,298 = -202,870 J/mole.
HW #6, P13.20 - Eeq as a Funtion of Temperature for Ammonia Synthesis from N2 and H2 - 14 pts
For the ammonia synthesis reaction :

The equilibrium conversion to ammonia is large at 300 K, but decreases rapidly with increasing T. However, reaction rates become appreciable only at higher temperatures. For a feed mixture of hydrogen and nitrogen in stoichiometric proportion :
a.) What is the equlibrium mole fraction of ammonia at 1 bar and 300 K ?
b.) At what temperature does the equilibrium mole fraction of ammonia equal 0.5 for a pressure of 1 bar ?
c.) At what temperature does the equilibrium mole fraction of ammonia equal 0.5 for a pressure of 100 bar, assuming the equilibrium mixture is an ideal gas ?
d.) At what temperature does the equilibrium mole fraction of ammonia equal 0.5 for a pressure of 100 bar, assuming the equilibrium mixture is an ideal solution of gases ?

The equilibrium conversion to ammonia is large at 300 K, but decreases rapidly with increasing T. However, reaction rates become appreciable only at higher temperatures. For a feed mixture of hydrogen and nitrogen in stoichiometric proportion :
a.) What is the equlibrium mole fraction of ammonia at 1 bar and 300 K ?
b.) At what temperature does the equilibrium mole fraction of ammonia equal 0.5 for a pressure of 1 bar ?
c.) At what temperature does the equilibrium mole fraction of ammonia equal 0.5 for a pressure of 100 bar, assuming the equilibrium mixture is an ideal gas ?
d.) At what temperature does the equilibrium mole fraction of ammonia equal 0.5 for a pressure of 100 bar, assuming the equilibrium mixture is an ideal solution of gases ?
HW #6, P13.15 - Isothermal Operation of an SO2 Catalytic Converter - 12 pts
The gas stream from a sulphur burner is composed of 15 mol% SO2, 20 mol% O2 and 65 mol% N2. This gas stream at 1 bar and 480oC enters a catalytic converter, where the SO2 is further oxidized to SO3. Assuming that the reaction reaches equilibrium, how much heat must be removed from the converter to maintain isothermal conditions ? Base your answer on 1 mole of entering gas.
HW #6, P13.13 - Effect of Pressure Change on Eeq of Reaction for the Hydrogenation of Acetaldehyde - 10 pts
The following reaction reaches equilibrium at 350oC and 3 bar:

If the system initially contains 1.5 mol H2 for each mole of acetaldehyde, what is the composition of the system at equilibrium ? What would be the effect of reducing the pressure to 1 bar ? Assume ideal gas behavior.

If the system initially contains 1.5 mol H2 for each mole of acetaldehyde, what is the composition of the system at equilibrium ? What would be the effect of reducing the pressure to 1 bar ? Assume ideal gas behavior.
HW #6, P13.5c - Problem title here - 6 pts
Consider the water-gas-shift reaction:
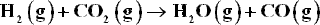
At high temperatures and low to moderate pressures, the reacting species form an ideal gas mixture. Application of the summability equation to Eq. (11.26) yields :

When the Gibbs energies of the elements in their standard states are set equal to zero, Gi = DGof,i for each species, and then :

At the beginning of Sec. 13.2, we noted that Eq. (14.64) is a criterion of equilibrium. Applied to the water-gas-shift reaction with the understanding that T and P are constant, this equation becomes :

Here, however, dn/dε = 0. The equilibrium criterion therefore becomes:

Once the yi are eliminated in favor of ε, Eq. (A) relates G to ε. Data for ΔGof,I for the compounds of interest are given with Ex 13.13. For a temperature of 1300 K (the reaction is unaffected by P) and for a feed of 1 mol H2 and 1 mol CO2:
a.) Determine the equilibrium value of ε by application of Eq. (B).
b.) Plot G vs. ε, indicating the location of the equilibrium value of ε determined in part (a).
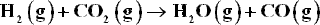
At high temperatures and low to moderate pressures, the reacting species form an ideal gas mixture. Application of the summability equation to Eq. (11.26) yields :

When the Gibbs energies of the elements in their standard states are set equal to zero, Gi = DGof,i for each species, and then :

At the beginning of Sec. 13.2, we noted that Eq. (14.64) is a criterion of equilibrium. Applied to the water-gas-shift reaction with the understanding that T and P are constant, this equation becomes :

Here, however, dn/dε = 0. The equilibrium criterion therefore becomes:

Once the yi are eliminated in favor of ε, Eq. (A) relates G to ε. Data for ΔGof,I for the compounds of interest are given with Ex 13.13. For a temperature of 1300 K (the reaction is unaffected by P) and for a feed of 1 mol H2 and 1 mol CO2:
a.) Determine the equilibrium value of ε by application of Eq. (B).
b.) Plot G vs. ε, indicating the location of the equilibrium value of ε determined in part (a).
Subscribe to:
Comments (Atom)